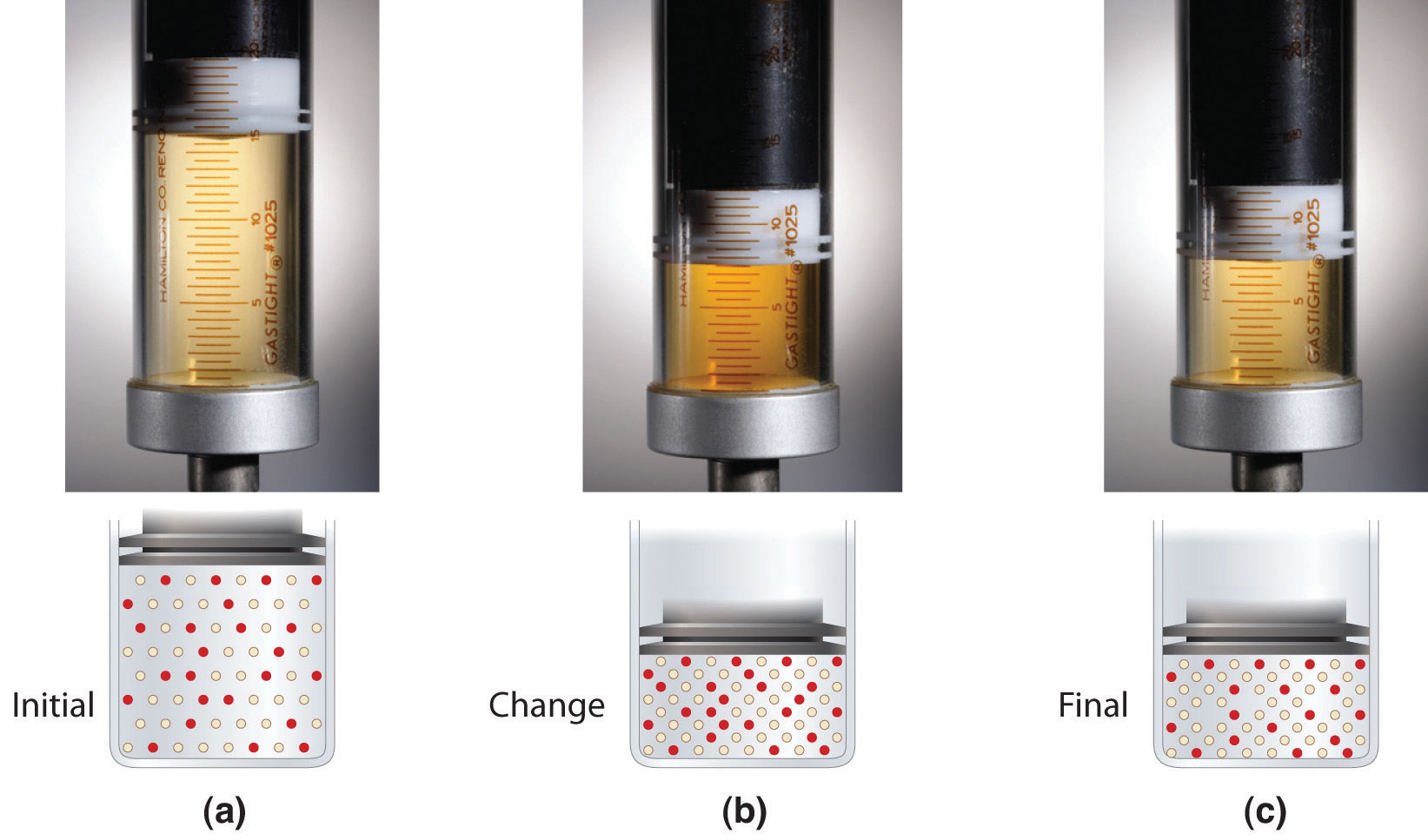
Gas Syringe Equilibrium . The two gases are different. The mixture is brown in colour. It is a dynamic equilibrium when the rate of the forward reaction equals the rate of the reverse. le chatelier's principle predicts that the equilibrium will shift to increase the concentration of products. a quick demonstration of the effect of pressure on dynamic equilibrium. The plunger is pulled out to reduce the pressure. the gas will expand and become darker as the equilibrium adjusts to the higher temperature by converting dinitrogen tetroxide to nitrogen. the equilibrium between no2 and n2o4 gases is set up in a gas syringe at room temperature. When carbon dioxide gas dissolves in water, it forms a weak acid solution due to the following reversible. a gas syringe contains a sample of an equilibrium mixture of the two gases.
from flatworldknowledge.lardbucket.org
a gas syringe contains a sample of an equilibrium mixture of the two gases. The two gases are different. le chatelier's principle predicts that the equilibrium will shift to increase the concentration of products. the equilibrium between no2 and n2o4 gases is set up in a gas syringe at room temperature. the gas will expand and become darker as the equilibrium adjusts to the higher temperature by converting dinitrogen tetroxide to nitrogen. It is a dynamic equilibrium when the rate of the forward reaction equals the rate of the reverse. The plunger is pulled out to reduce the pressure. The mixture is brown in colour. When carbon dioxide gas dissolves in water, it forms a weak acid solution due to the following reversible. a quick demonstration of the effect of pressure on dynamic equilibrium.
Chemical Equilibrium
Gas Syringe Equilibrium When carbon dioxide gas dissolves in water, it forms a weak acid solution due to the following reversible. The mixture is brown in colour. The two gases are different. the gas will expand and become darker as the equilibrium adjusts to the higher temperature by converting dinitrogen tetroxide to nitrogen. a quick demonstration of the effect of pressure on dynamic equilibrium. le chatelier's principle predicts that the equilibrium will shift to increase the concentration of products. When carbon dioxide gas dissolves in water, it forms a weak acid solution due to the following reversible. The plunger is pulled out to reduce the pressure. It is a dynamic equilibrium when the rate of the forward reaction equals the rate of the reverse. the equilibrium between no2 and n2o4 gases is set up in a gas syringe at room temperature. a gas syringe contains a sample of an equilibrium mixture of the two gases.
From courses.lumenlearning.com
Chemical Equilibria General Chemistry Gas Syringe Equilibrium It is a dynamic equilibrium when the rate of the forward reaction equals the rate of the reverse. The plunger is pulled out to reduce the pressure. the equilibrium between no2 and n2o4 gases is set up in a gas syringe at room temperature. The two gases are different. The mixture is brown in colour. a gas syringe. Gas Syringe Equilibrium.
From www.researchgate.net
Reproducibility of syringe equilibrium / headspace gas chromatography Gas Syringe Equilibrium the gas will expand and become darker as the equilibrium adjusts to the higher temperature by converting dinitrogen tetroxide to nitrogen. the equilibrium between no2 and n2o4 gases is set up in a gas syringe at room temperature. The two gases are different. The mixture is brown in colour. The plunger is pulled out to reduce the pressure.. Gas Syringe Equilibrium.
From www.researchgate.net
The syringe and the method of taking the required volume from the gas Gas Syringe Equilibrium a gas syringe contains a sample of an equilibrium mixture of the two gases. the equilibrium between no2 and n2o4 gases is set up in a gas syringe at room temperature. The mixture is brown in colour. the gas will expand and become darker as the equilibrium adjusts to the higher temperature by converting dinitrogen tetroxide to. Gas Syringe Equilibrium.
From www.researchgate.net
Reproducibility of syringe equilibrium / headspace gas chromatography Gas Syringe Equilibrium The two gases are different. It is a dynamic equilibrium when the rate of the forward reaction equals the rate of the reverse. When carbon dioxide gas dissolves in water, it forms a weak acid solution due to the following reversible. The mixture is brown in colour. le chatelier's principle predicts that the equilibrium will shift to increase the. Gas Syringe Equilibrium.
From www.chegg.com
Solved ID A 19. A full syringe contains this equilibrium 2 Gas Syringe Equilibrium a quick demonstration of the effect of pressure on dynamic equilibrium. When carbon dioxide gas dissolves in water, it forms a weak acid solution due to the following reversible. It is a dynamic equilibrium when the rate of the forward reaction equals the rate of the reverse. The plunger is pulled out to reduce the pressure. The two gases. Gas Syringe Equilibrium.
From dxokvwiuo.blob.core.windows.net
What Is A Gas Syringe at Juan Palmer blog Gas Syringe Equilibrium When carbon dioxide gas dissolves in water, it forms a weak acid solution due to the following reversible. The two gases are different. the equilibrium between no2 and n2o4 gases is set up in a gas syringe at room temperature. le chatelier's principle predicts that the equilibrium will shift to increase the concentration of products. the gas. Gas Syringe Equilibrium.
From www.youtube.com
7.1 using a gas syringe to measure the speed of reaction YouTube Gas Syringe Equilibrium a quick demonstration of the effect of pressure on dynamic equilibrium. le chatelier's principle predicts that the equilibrium will shift to increase the concentration of products. the equilibrium between no2 and n2o4 gases is set up in a gas syringe at room temperature. The mixture is brown in colour. It is a dynamic equilibrium when the rate. Gas Syringe Equilibrium.
From gootutorials.blogspot.com
How To Use A Gas Syringe To Measure Gas Gas Syringe Equilibrium a quick demonstration of the effect of pressure on dynamic equilibrium. the equilibrium between no2 and n2o4 gases is set up in a gas syringe at room temperature. le chatelier's principle predicts that the equilibrium will shift to increase the concentration of products. The plunger is pulled out to reduce the pressure. The two gases are different.. Gas Syringe Equilibrium.
From www.alovie.com
Glass Syringes Gas Syringe Equilibrium The plunger is pulled out to reduce the pressure. The mixture is brown in colour. a quick demonstration of the effect of pressure on dynamic equilibrium. le chatelier's principle predicts that the equilibrium will shift to increase the concentration of products. a gas syringe contains a sample of an equilibrium mixture of the two gases. It is. Gas Syringe Equilibrium.
From www.youtube.com
Oxygen in air gas syringes and copper method YouTube Gas Syringe Equilibrium When carbon dioxide gas dissolves in water, it forms a weak acid solution due to the following reversible. a gas syringe contains a sample of an equilibrium mixture of the two gases. The two gases are different. a quick demonstration of the effect of pressure on dynamic equilibrium. the gas will expand and become darker as the. Gas Syringe Equilibrium.
From www.youtube.com
Chemistry Unit 3 Demonstration of equilibrium in a syringe YouTube Gas Syringe Equilibrium The two gases are different. The mixture is brown in colour. le chatelier's principle predicts that the equilibrium will shift to increase the concentration of products. a quick demonstration of the effect of pressure on dynamic equilibrium. It is a dynamic equilibrium when the rate of the forward reaction equals the rate of the reverse. the equilibrium. Gas Syringe Equilibrium.
From www.alamy.com
Chemical equilibrium experiment. Image 2 of 2. Student applying Gas Syringe Equilibrium When carbon dioxide gas dissolves in water, it forms a weak acid solution due to the following reversible. the gas will expand and become darker as the equilibrium adjusts to the higher temperature by converting dinitrogen tetroxide to nitrogen. It is a dynamic equilibrium when the rate of the forward reaction equals the rate of the reverse. The two. Gas Syringe Equilibrium.
From www.scribd.com
Form 2 Chapter 6 Syringe Gases Gas Syringe Equilibrium The mixture is brown in colour. The two gases are different. When carbon dioxide gas dissolves in water, it forms a weak acid solution due to the following reversible. the gas will expand and become darker as the equilibrium adjusts to the higher temperature by converting dinitrogen tetroxide to nitrogen. a gas syringe contains a sample of an. Gas Syringe Equilibrium.
From www.numerade.com
SOLVED A gas syringe at 20^∘ C contains 20.0 mL of CO2 gas. The Gas Syringe Equilibrium The two gases are different. the equilibrium between no2 and n2o4 gases is set up in a gas syringe at room temperature. The plunger is pulled out to reduce the pressure. a gas syringe contains a sample of an equilibrium mixture of the two gases. le chatelier's principle predicts that the equilibrium will shift to increase the. Gas Syringe Equilibrium.
From www.youtube.com
Using a gas syringe to measure volumes of gases YouTube Gas Syringe Equilibrium The mixture is brown in colour. It is a dynamic equilibrium when the rate of the forward reaction equals the rate of the reverse. the equilibrium between no2 and n2o4 gases is set up in a gas syringe at room temperature. a quick demonstration of the effect of pressure on dynamic equilibrium. the gas will expand and. Gas Syringe Equilibrium.
From www.researchgate.net
Reproducibility of syringe equilibrium / headspace gas chromatography Gas Syringe Equilibrium a gas syringe contains a sample of an equilibrium mixture of the two gases. When carbon dioxide gas dissolves in water, it forms a weak acid solution due to the following reversible. the gas will expand and become darker as the equilibrium adjusts to the higher temperature by converting dinitrogen tetroxide to nitrogen. The mixture is brown in. Gas Syringe Equilibrium.
From flatworldknowledge.lardbucket.org
Chemical Equilibrium Gas Syringe Equilibrium the gas will expand and become darker as the equilibrium adjusts to the higher temperature by converting dinitrogen tetroxide to nitrogen. It is a dynamic equilibrium when the rate of the forward reaction equals the rate of the reverse. The plunger is pulled out to reduce the pressure. le chatelier's principle predicts that the equilibrium will shift to. Gas Syringe Equilibrium.
From www.researchgate.net
The syringe and the method of taking the required volume from the gas Gas Syringe Equilibrium When carbon dioxide gas dissolves in water, it forms a weak acid solution due to the following reversible. It is a dynamic equilibrium when the rate of the forward reaction equals the rate of the reverse. the equilibrium between no2 and n2o4 gases is set up in a gas syringe at room temperature. The mixture is brown in colour.. Gas Syringe Equilibrium.